



What Is Medical Device Registration?
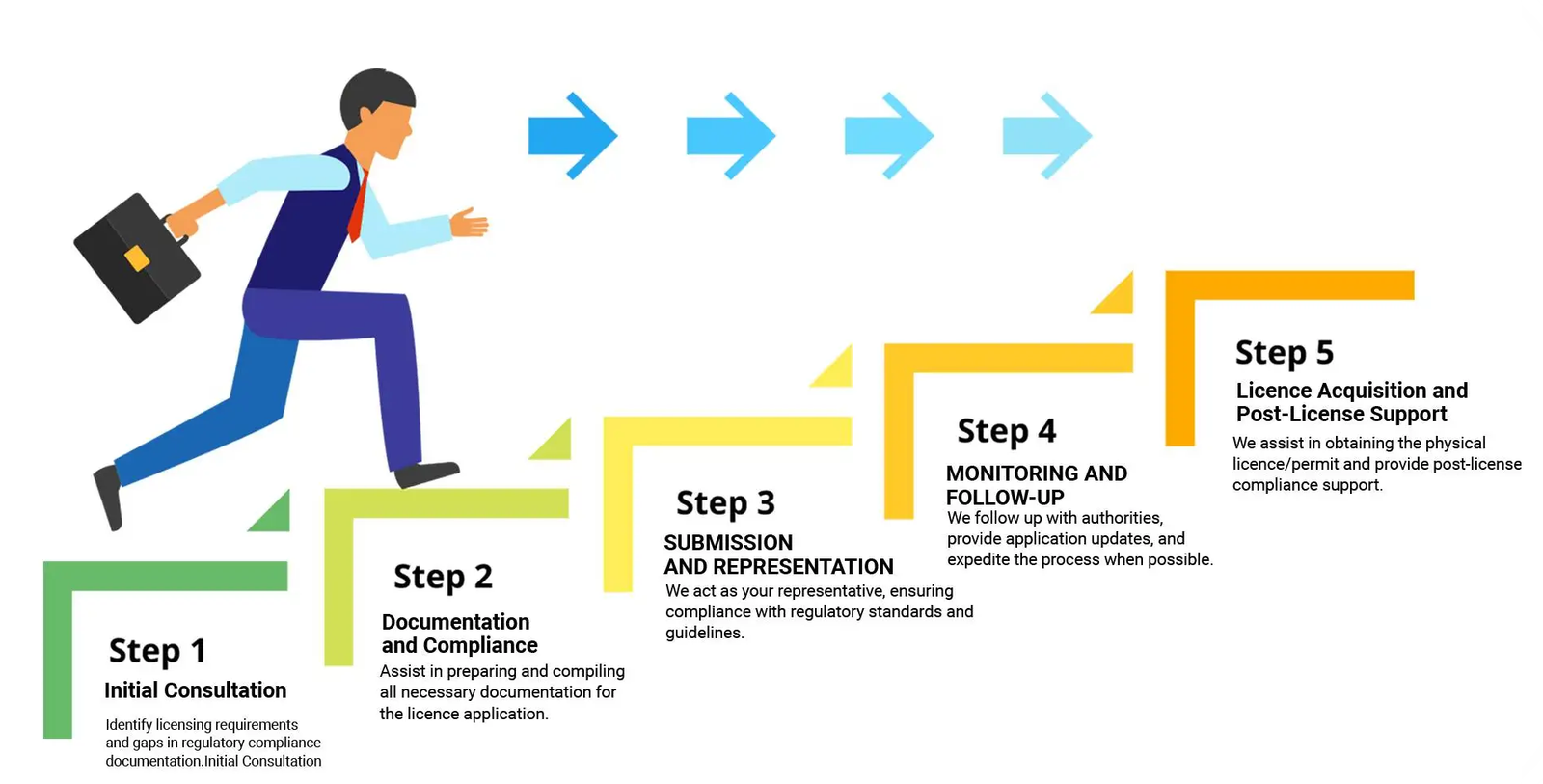
Medical device registration is a regulatory process that includes the submission of information and documents to a government agency and regulatory authority. The process is done to obtain approval or authorization to market and distribute medical devices in a particular jurisdiction.
Medical devices include a wide range of products such as diagnostic equipment, surgical instruments, implantable devices, in vitro diagnostic devices, and more.
The medical device registration is necessary because medical devices on the market need certain safety, efficacy, quality standards, and they are approximately labelled and manufactured. The medical device registration process varies from country to country and is governed by national and international regulations.