Vast experience, extensive tie ups PAN india, Hand holding support from Launch till onboarded for sale in market. Strategy making for CRO and its related legal aspects, representations before CDSCo, availing international references, participant of IRAQ’s which enable us to keep broadening our experience and outlook for the medical device industry – depth and breadth.
A CRO that focuses specifically on the development, clinical testing, and Data management of medical devices is known as a medical device CRO.
Contract Research Organisation (CRO), an Organisation contracted by the sponsor(a manufacturer of medical devices) to perform clinical trial-related duties and functions, has been present globally since its existence in the 1930s.
CROS are of various sizes, providing research and support services to pharmaceutical, biotechnological, and health companies within and outside the domains of good laboratory practice (GLP), good manufacturing practice (GMP), and good clinical practice (GCP) CROs are key players in clinical research as they possess the knowledge and capabilities needed in developing and conducting clinical research.
Working with a CRO helps to save time and money while ensuring the quality of these trials in compliance with the regulations.
1. Preclinical research activities
2. Select in SMO (Site Management organisation)
3. Protocol design
4. Clinical trial management
5. Investigator’s brochure
6. Clinical Study Reports
CROS help to ensure the trial is recorded, conducted, and reported in compliance with the protocol, regulatory requirements, and the International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH GCP), site personnel training, and pharmacovigilance.
Regulatory submissions
CROs help to ensure compliance with regulatory and ethics requirements by preparing and submitting regulatory materials or documents to regulatory agencies. CROS are also involved.
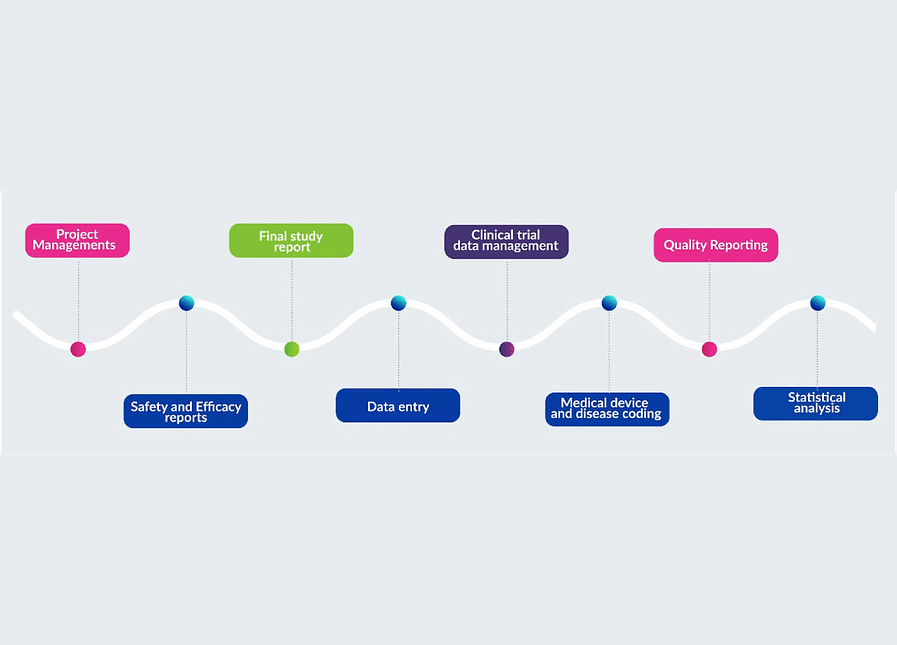


Sponsors who want to conduct a clinical trial hire CROs, who play an important role. They provide a wide range of services related to the study’s conduct from beginning to end. The success of your trial will depend on the CRO’s management and capabilities, so choosing a good CRO like ELT is Important.