Bureau of Indian Standards (BIS) Certification holds immense significance in ensuring quality and compliance for products circulating within India. Established under the Bureau of Indian Standards Act, of 1986, Including multiple equipment it is necessary to apply BIS Certificate For Medical Devices. BIS is the national body that formulates standards and certifies products for quality, safety, and reliability. It ensure the cosnumer about the safety &quality of the product that they consume. It is mandatory to apply for the medical devices for any class. Without this BIS approval a manufacturer or importer of medical device is not allow to regulate products in market.
So, ELT Corporate is here to assist you to apply for BIS Certificate For Medical Devices or equipments whether you are manufacturer or importer. Simply you have to connect with us and our team is ready to assist you.
The Bureau of Indian Standards (BIS) in India is functioning under Ministry of Consumer Affairs. It is responsible for grant of BIS certification. The BIS certification signifies a third party, i.e., BIS, assuring to customers the quality, dependability, and safety of any product. The BIS is involved in a number of activities, such as product certification, quality control, testing, hallmarking, calibration schemes, etc.
BIS is necessary to provide the safeguard to the public health and quality assurance to the consumers. It simply protects the consumers from the hazardous products and promotes the consumer confidence to consumer goods without having any doubts.
The BIS certificate for medical device is necessary for the Manufacturer & Importer of medical device. Because is important for all the consumers, it shows that your product is safe to consumer.

Yes, BIS Registration for medical device is mandatory to have without this license you are not allowed to regulate your products in indian market. It ensure about the quality and safety of the products that they are consuming is safe & approved by the government.
The Bureau of Indian Standards’ role in medical devices is to maintain the healthcare system and keep an eye on the calibre of equipment used in the medical industry to ensure that it is used appropriately and per BIS-approved safety protocols. The medical device role is utilised as per set indian standards of a technical committee established by BIS that the work under the medical equipment, hospital planning department, for such as medical devices, i.e. CT, i.e. scan, PET Scan, MRI, X-ray, weight machine, medical laboratory equipment, research laboratory, surgical equipment, veterinary products need the BIS certificate.
The BIS registration for medical device is as follows:
NOTE – If the inspector gets satisfied with all the documents and site plant then the BIS Certificate is grant by the BIS department. If your application cancelled then you must connect with the BIS Consultancy Expert to resolve all the issues.



Basically there are various types of BIS certifications available but if we are talking about BIS License for medical devices, then there are two certifications for BIS available. That is “ISI (Indian Standard Institute)” and “FMCS (Foreign Manufacturing Certification Scheme)”.
A certificate program called ISI certification is available to Indian manufacturers working on goods that fall under BIS’s purview. It assures that a product complies with Indian safety requirements. To ensure that a product complies with Indian standards, FMCS is, however, applicable to foreign manufacturers. Although the products covered by both certification programmes are somewhat comparable, their certification procedures vary wildly.
Compared to FMCS, the ISI certification process is much simpler because it involves more on-site activities, such as client travel to their destination country, customs clearance, product delivery to India, and other things. FMCS is comprehensive because of these off-shore activities that it incorporates.
Application Filling >> Industry Inspection >> Sample Testing >> Product Test Report Review >> ISI Certificate Grant Figure
Here is the list of needed documents for BIS Certification for medical devices ISI in India:-
There are many benefits of ISI Certificate Registration, such as whether products meet quality standards or not. To understand this concept, we have mentioned all the pointers as the following:-
FMCS is known as a Foreign Manufacturer Certification scheme. The program from the Bureau of Indian Standards, a short BIS, allows foreign manufacturers to register their products for quality under the ISI mark in India. You can easily understand it as the FMCS Certification helps foreign companies prove that their products meet Indian safety and quality standards.
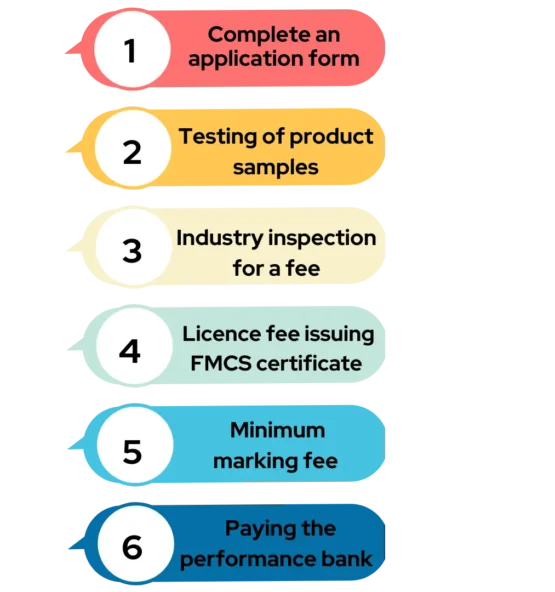
Fill the Application >> Testing of Product Sample >> Inspection for Fee Verification by Industry >> License fee Issuing FMCS Certificate >> Minimum Marking Fee
To obtain BIS for medical devices FMCS in India you will required these documents:-
FMCS offers various benefits for Foreign Manufacturers, who are seeking to export their products in India.
Only 38 different kinds of medical equipment must currently obtain an import licence (Form MD-15) from India’s Central Drugs Standard Control Organisation (CDSCO) before being sold. All risk Class A and B medical devices will need to have an import licence as of October 1, 2022. The same requirement will apply to all risk Class C and D medical devices. All non-notified medical devices must be registered in the interim. India’s licencing requirements for medical devices include:
ELT Corporate is a team of experts that guide you in entire process for filing an application for BIS registration of medical devices. The team will provide you all information and education that is required on all aspects of BIS Certification. We will provide information about whether the license is required for the products or not. The team will guide you to analyze the products requirements and specification. The aim of our company and team is to provide expect timely & quality service delivery to our clients.
BIS Certifications are valid for two years According to factory inspection and product testing, BIS is a body that ensures that a product must adhere to the rules and issues certificates.
YES, BIS registration for medical devices is applicable for all class of medical equipments.
The BIS certificate is required to show that the product is safe to consumer for the users. It indicates that the products meets all the Indian standards to regulate in market.
The timeline of the BIS registration approval is depend on the products. Usually the whole process takes 5 to 6 months.