Sustainability practices in medical device manufacturing focus on creating healthcare products in a way that minimizes environmental damage, conserves natural resources, and ensures long-term safety for both patients and the planet. With rising demand for medical devices worldwide, manufacturers are under increasing pressure to balance product safety, regulatory compliance, and sustainability. Practices such as energy efficiency, waste reduction, sustainable packaging, and eco-friendly materials are now becoming essential in medical device production.
What Does Sustainability Practices In Medical Device Manufacturing Mean?
Sustainability practices in medical device manufacturing mean adopting methods that reduce environmental harm, conserve resources, and create devices responsibly. This includes:
- Using eco-friendly raw materials.
- Reducing energy and water consumption.
- Recycling waste generated during production.
- Designing packaging that reduces plastic and non-biodegradable waste.
It ensures that medical devices are manufactured in a way that supports both healthcare needs and environmental protection.
Why Are Sustainability Practices In Medical Device Manufacturing Important?
Sustainability practices in medical device manufacturing are important because:
- They reduce carbon emissions and help fight climate change.
- They protect patient health by avoiding harmful materials.
- They lower costs in the long run by saving energy and materials.
- They ensure global compliance with eco-friendly regulations.
- They build brand trust, as consumers increasingly prefer sustainable companies.
Sustainability Practices in Medical Device Manufacturing (1-Min Explainer)
What Environmental Challenges Do Manufacturers Face In Sustainability Practices In Medical Device Manufacturing?
Manufacturers face several environmental challenges, such as:
- High plastic usage in disposable medical devices.
- Energy-intensive production processes.
- Non-biodegradable packaging materials.
- Medical waste disposal, which is often hazardous.
- Limited recycling infrastructure for healthcare products.
These challenges make it difficult to balance product safety with sustainability goals.
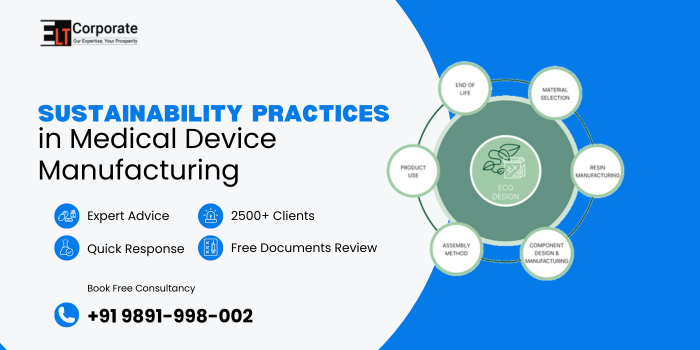
Which Materials And Raw Resources Are Most Relevant For Sustainability Practices In Medical Device Manufacturing?
Key materials that support sustainability include:
- Bioplastics made from renewable sources like corn starch or sugarcane.
- Recyclable metals such as stainless steel and aluminum.
- Glass in place of plastics for some device parts.
- Paper-based or compostable packaging instead of plastic.
- Latex-free alternatives to reduce allergies and environmental harm.
Choosing sustainable materials helps reduce environmental footprints and promotes circular economy.
How Can Energy Efficiency Be Improved Under Sustainability Practices In Medical Device Manufacturing?
Manufacturers can improve energy efficiency by:
- Switching to renewable energy sources like solar or wind.
- Using energy-efficient machinery in production lines.
- Implementing automation and AI to reduce energy wastage.
- Reusing waste heat from production.
- Regularly auditing energy usage.
Energy efficiency reduces costs and helps meet international green manufacturing standards.
What Waste Reduction And Recycling Methods Are Part Of Sustainability Practices In Medical Device Manufacturing?
Waste reduction is a core part of sustainability practices in medical device manufacturing. Methods include:
- Recycling packaging waste such as cardboard and plastics.
- Reprocessing single-use devices where regulations allow.
- Reducing scrap material through precision manufacturing.
- Designing for reuse, such as rechargeable devices.
- Partnering with waste management companies for safe disposal of biomedical waste.
How Does Sustainable Packaging Fit Into Sustainability Practices In Medical Device Manufacturing?
Sustainable packaging plays a vital role in medical device manufacturing by:
- Using biodegradable and recyclable materials.
- Reducing the size and weight of packaging to save resources.
- Designing minimalist packaging to cut down plastic.
- Following international eco-labeling standards.
This not only reduces waste but also improves brand image and meets compliance requirements.
What Certifications And Standards Support Good Sustainability Practices In Medical Device Manufacturing?
Some key certifications include:
- ISO 14001 – Environmental management system.
- ISO 13485 – Quality management for medical devices with sustainability integration.
- RoHS Compliance – Restriction of hazardous substances.
- EPEAT & ENERGY STAR – Standards for energy efficiency.
- CE Marking (Europe) – Ensures compliance with environmental safety.
What Are The Regulatory Or Compliance Risks Related To Sustainability Practices In Medical Device Manufacturing?
Risks include:
- Non-compliance penalties for using banned materials.
- Product recalls if packaging or waste handling does not meet standards.
- Import/export restrictions in regions with strict environmental laws.
- Loss of certifications such as CE or ISO.
Not following sustainability rules can harm business reputation and limit market access.
How Can Manufacturers Measure And Report On Their Sustainability Practices In Medical Device Manufacturing?
Manufacturers can measure and report using:
- Carbon footprint tracking tools.
- Sustainability audits.
- Publishing annual sustainability reports.
- Tracking energy, water, and material usage.
- Using life-cycle assessments (LCA) to measure device impact from production to disposal.
Transparent reporting improves trust among regulators, customers, and stakeholders.
What Role Does Innovation Play In Scaling Sustainability Practices In Medical Device Manufacturing?
Innovation is a driving force in sustainability. Examples include:
- 3D printing to reduce material waste.
- Smart manufacturing with IoT and AI.
- Green chemistry for safer production processes.
- Biodegradable device design.
- Circular economy models where products are reused or repurposed.
Why Is Professional Consultancy Important For Implementing Sustainability Practices In Medical Device Manufacturing Effectively?
Professional consultancy is crucial because:
- Experts understand complex global sustainability regulations.
- They help in choosing eco-friendly materials and suppliers.
- Consultants assist in certification processes like ISO 14001 and CE.
- They provide strategies for waste reduction, energy efficiency, and reporting.
- They guide companies in balancing cost-effectiveness with compliance.
By working with consultants, companies save time, avoid penalties, and ensure their devices meet both healthcare and environmental standards.
What is the biggest benefit of sustainability practices in medical device manufacturing?
It helps reduce environmental impact while ensuring safe, high-quality medical devices.
Are sustainability practices in medical device manufacturing costly?
Initially, costs may rise, but in the long run they reduce expenses through energy savings, better resource use, and fewer compliance risks.

Comments are closed