We Are ELT Corporate
Medical Device Manufacturing License & ISO 13485(QMS) Certification Consultant
Want to manufacture Medical Devices but unsure where to start?
Welcome to ELT Corporate – Your Trusted Partner for CDSCO Registration and Medical Device Certification.
- MD Manufacturing Registration Post Approval Changes
- Experts in CDSCO Query Handling
- Manufacturing Site Inspection
- MD Manufacturing Registration Endorsement
- Device & Plant Master File Preparation
- MD Manufacturing Registration Retention
- MD Manufacturing Test License
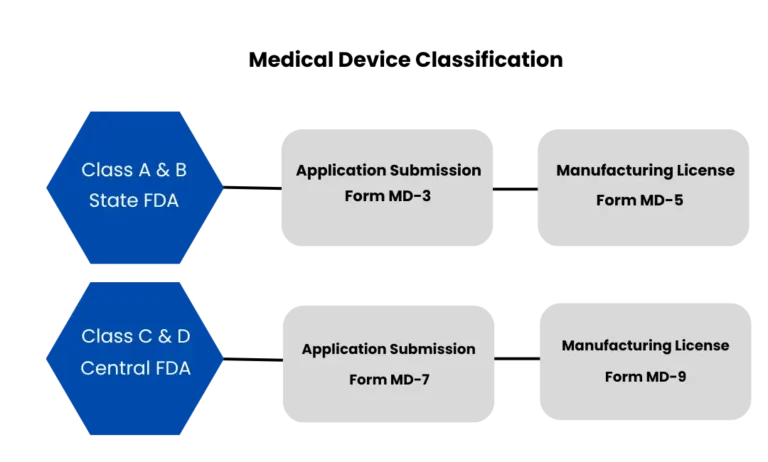
Overview Medical Device Manufacturing License (MD 5/MD 9)
A Manufacturing License for medical devices in India is an official permission granted by the Central Drugs Standard Control Organization (CDSCO), which is under the Ministry of Health and Family Welfare.
This license is mandatory for any company intending to manufacture medical devices, including implants, diagnostic equipment, surgical instruments, and other related products. MD5 and MD9 are licenses for manufacturing medical devices in India. The MD5 license is for Class A and Class B medical devices, and it is granted by the State Licensing Authority (SLA). The MD9 license is for Class C and Class D devices, and it is granted by the Central Licensing Authority (CLA).

ISO 13485 : 2016 -Quality Management System
ISO 13485:2016 certification is an international standard for Quality Management Systems (QMS) in the medical device industry. It helps companies ensure their products are safe, effective, and meet regulatory requirements. Getting certified shows that your business follows best practices for quality, improving customer trust and opening up new market opportunities. ISO 13485 focuses on managing risks, improving processes, and ensuring continuous quality. Our team can help guide you through the certification process and ensure your business meets the standard. Contact us today to get started.
IVD In-Vitro Diagnostic Device Manufacturing License
An In-Vitro Diagnostic (IVD) Device Manufacturing License is a regulatory authorization required for companies that manufacture IVD medical devices in India. This license is issued by Central Drugs Standard Control Organization(CDSCO). This license is mandatory for both domestic and foreign manufacturers who want to legally manufacture IVD medical device in India. IVDs are used to collect, prepare, and test samples from the human body.It ensures that devices like diagnostic kits, pregnancy tests, blood glucose meters, and lab instruments.
IVD import license
Medical Devices & Classification Based on Risk Level
Class A
- Surgical & Nitrile Gloves
- Thermometer
- Surgical Masks & Gown
- N95 Mask & PH Meter
- CryoCentrifuge
- Sample Slides
- Slide Scanner
- Scissors & Forceps
- Artificial Hand & Knee
- OT Table & Light
- Tongue Depressors
- Surgical Microscope
Class B
- Hypodermic Needles
- Infusion Sets
- Syringes
- Gauze Swab
- Oxygen Concentrator
- Ice Lined Refrigerator
- Hematolozy Analyzer
- Glucometer Strips
- Spirometer
- Sphygmomanometer
- Fetal Doppler
- Hearing Aid
Class C
- Implants
- Haemodialysis Catheter
- Bone Plates and Screws
- Patient Monitor & ECG
- Infusion & Syringe Pump
- Nebulizer
- CPAP & BPAP
- PaceMaker
- OutPut Monitor
- Defibrillator & Autoclave
- Ventillator
- Anesthesia Workstation
Class D
- Heart Valve
- Bone Plates and Screws
- Implantable Pacemaker Pulse Generator
- Electronic Epidural Space Locator Control Unit
- Spinal Needle Bioimpedance Navigation Unit
- Ventricular Bypass Device
- Carotid Sinus Nerve Stimulator
- Fistula-Repair Biomatrix Implant
- Tympanic Membrane Contact Hearing Aid
Benefits Of Manufacturing License Medical Device
Who can Apply For Medical Device Manufacturing License?

New Manufacturers
Any person who wants to start a manufacturing business of medical Device
Importer & Manufacturer
Any existing importer or Manufacturer who is willing to start medical device business.

Authorized Representative
Any authorised person on the behalf of Manufacturer or importer
Documents Required For Medical device Manufacturing License
Following are the key documents an applicant must submit to obtain the medical device manufacturing license, in accordance with the CDSCO, Medical Device Rules, 2017:
- Cover Letter
- Application Form mentioning all the details of applicant and medical device
- ISO 13485 Certification (for quality management system)
- Risk Management Plan
- Clinical Evaluation Report
- Labels and IFU (Instructions for Use)
- Usage Instruction and Warnings
- Payment Receipt (TR6 Challan)
- Power of Attorney Certificate
- CE Design certificate
- Undertaking of Authenticity of Information Provided
- Quality Assurance Certificate
- Declaration of Conformity
- Schedule D(I) Documentation
- Device Master File (DMF)
- Plant Master Report
- Free Sale Certificate (FSC)
Process of Medical Device Manufacturing License
Phase I

Phase II

Phase III

Phase IV

Our Genuine Trusted Partners & Clients









ELT CORPORATE
Why Choose Us As An Expert Advisor?
Choosing ELT Corporate as An Expert Advisor Offers several reasons:-
Expertise
ELT Corporate have a team of professionals with deep knowledge in various fields.
Customized Solutions
To meet your specific business we provide tailored solutions to the clients.
Compliance
We aim to ensure that your business complies with all the relevant regulations and standards.
Efficiency
The streamlined process of our experts improves efficiency and reduces costs.
Support
ELT Corporate offers ongoing support and guidance to help your business grow and succeed.
Frequently Asked Questions(FAQs)
The process involves a documentation review, internal audits, and an external audit by an accredited body. We’ll guide you through each step to ensure compliance.
Generally, ISO Certification may take time between 3-6 months, based on the complexity and size of the business and the ISO standard.
The costs vary based on the size of your organization and the complexity of the certification or license, but we offer competitive pricing and transparent billing.
Our Testimonials
Get in Touch with Us
- +91 8750013240
- info@medicaldeviceregistration.com
- ELT House No. 271, D-15, Sec-3, Rohini, New Delhi-110085
© 2024 – ELT Corporate Pvt Ltd. | All rights reserved.



