With growing demand for healthcare products, the indian government has made strict regulations in place to provide safety & quality of medical devices. According to these regulations, it is obligatory to obtain MD 41 and MD 42 licenses for individuals who are engaged in the wholesale distribution of medical devices in India. If you are a distributor or supplier wanting to sell medical devices, you should know about these licenses to comply with the CDSCO.
This manual will take you through all the information regarding MD 41 and 42, including the registration process, eligibility, and validity.
What is an MD 41 and MD 42 License?
MD 41 & 42 are the important legal documents required by businesses and individuals engaged in the wholesale business of medical devices. This license is governed by the Central Drugs Standard Control Organization under the Medical Device Rule, 2017. For better understanding, we have defined each form below.
MD 41: Application form used to request the registration certificate for selling, stocking, exhibiting, or distributing medical devices and IVDs.
MD 42: The actual license issued by the State Licensing Authority (SLA) after successful submission and inspection under Form MD 41.
Why Is It Important To Obtain MD 41 & MD 42 licenses?
MD 41 & MD 42 are important if you are a wholesaler, retailer, and stockist of medical devices, and this license helps build trust in the products you sell, safe and quality medical devices. The further more importance of this MD 42 license is as follows:
- Regulatory Compliance – Through this it ensure that the imported medical devices meets the standards set by the CDSCO (Central Drugs Standard Control Organization). It is mandatory for all the medical device distributor to ensure that they are working as per the regulatory standards of indian laws. Without this license you are not allowed to distrribute any medical devices. If you do this then it is a illegal activity.
- Ensuring Public Safety – One of the primary reason to obtain the license of MD 42 is to ensure the public safety. It ensures that the product is safe to use and selling under the section of CDSCO guidenlines.
- Quality Control and Standards – The license guarantees that the medical devices being imported are subject to rigorous scrutiny to ensure high quality and efficacy. This ensures that the device meet the both international and national quality standards.
- Building Trust in the Market – When you obtain a license then this will help you to build a trust in the market and boost number of sales. It ensures the consumer that the product is safe to use and authorized by regulatory authorities, ensuring that the products meet all the legal and safety requirements.
- Business Expansion and Opportunities – This will help you to boost the business expansion and opportunities to access large indian healthcare market. this license allows foreign manufacturers and distributors to establish themselves in the market while complying with Indian laws and regulations.
- Legal Protection – When you obtain this MD 41 and MD 42 license then this will ensures legal protection to businesses by ensuring they are operating within the bounds of Indian laws. It helps the business to avoid penalties, fines and onfiscation of products for operating without the required licenses.
How To Apply For an MD 42 License?
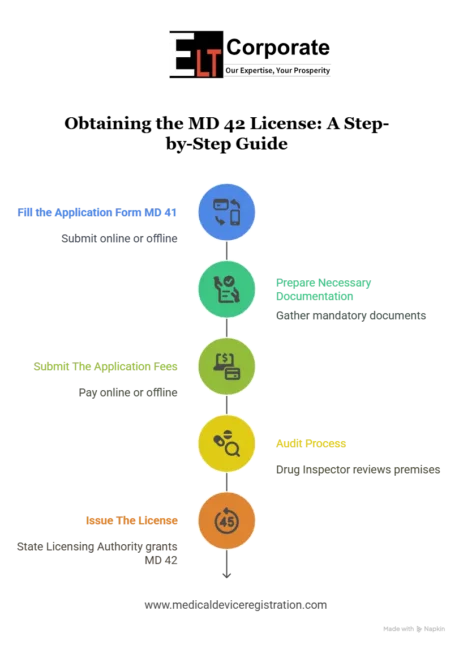
To obtain the MD 42 license, you need to follow the basic procedure in order to comply with regulatory standards. The steps to obtain a license are as follows:
1. Fill the Application Form MD 41
First, you need to fill out an application for MD 41 and submit it online or offline. Some states have their own online portals for filing MD 41 [i.e., Nivesh Mitra (U.P.), CDSCO (Gurugram)]. But most of the states have offline processes means you need visit State FDA office and submit the application.
2. Prepare Necessary Documentation
As mentioned below list attach all the required documents. The documents are mandatory to attach if you omit any of the documents then your application must be rejected.
3. Submit The Application Fees
To obtain the license, you must submit the application fee as per the government guidelines. You can simply submit the application fee in two ways: online or offline.
4. Audit Process
After submitting the MD 41 application, a Drug Inspector will review your premises. If you go with the offline process, then the Drug Inspector may visit your location for an inspection. For the audit of the application, you need to visit the FDA office for document verification.
5. Issue The License
When the drug inspector finds that all things are as per the guidelines, they will issue the MD 42 license. If they find any issue, then they will conduct an inquiry or inform the concerned business.
NOTE – Primarily, the State Licensing Authority Issues the MD 42 License after checking all the important items. Furthermore, when convinced that the applicant has followed all the rules and regulations of Medical Devices, the license is provided in Form 42.
What Documents are Required for an MD 42 License?
Documents that are needed to obtain the MD 42 license are mentioned in the list below:
- Completed Form MD-41
- Cover letter with business intent
- Floor/site layout of your premises
- Rent agreement or property proof
- Storage facility details (refrigeration if applicable)
- Employee qualifications and resume
- Academic certificates of the competent technical staff
- Affidavit of appointment
- Declaration of regulatory compliance
Apart from that, you may also need Documents like GST, Trade License, Premise Plan, etc.
What is the Government Fee for an MD 42 License?
The government fee for the MD 42 is Rs. 3000, which is required from everyone applying for it. But it’s liable to change according to modifications in updates, so directly talk to the experienced in the field.
Who Needs To Fill MD 42 License?
Any person who is engaged in the wholesale selling, distribution, or stocking of medical devices in India. Whether you are a distributor, reseller, or importer, you must have the MD 42 License.
To acquire it, they are first required to apply through Form MD 41, and when approved, the MD 42 license is issued.
What Is The Renewal Cost For the MD 42 License?
The Client has to pay Rs. 3000 for the renewal of the Medical Device Registration Certificate, MD 42.
Who Qualifies As Competent Technical Staff For MD 42 Registration Service?
- A person who holds a degree from a recognized University/Institution, who is a registered pharmacist, or who has passed the intermediate examination with one year of experience in dealing with the sale of medical devices.
- The Candidate should appoint a Competent Technical Staff to direct and supervise the sales activity of medical devices. The Competent Technical Staff must hold the above educational qualifications and experience.
Why Amazon, Flipkart, and other E-Commerce Portals are Asking for an MD 42 License?
Amazon and Flipkart are requesting an MD 42 license, which is required to sell medical equipment in India. All medical devices imported, produced, or marketed in India require an MD 42 license. The CDSCO issued this license.
How ELT Can Help You?
ELT Corporate can assist you in obtaining your MD 41 & MD 42 licenses hassle-free by taking care of the entire process. This includes document preparation and application filing to regulatory compliance and follow-ups with CDSCO or State Licensing Authorities.
Under professional supervision, ELT Corporate provides a fast, flawless, and hassle-free experience of licensing for your medical device company.
What is a Wholesale License for Medical Devices in India?
A Wholesale License for Medical Devices in India is a legal permit issued by CDSCO or State Authorities to sell, stock, or distribute medical devices in bulk.
It is obtained by applying through Form MD-41 and granted as Form MD-42 under the Medical Devices Rules, 2017.
Is a Drug License Required for Medical Devices?
In India, only registered medical devices are regulated as pharmaceuticals under the Pharmaceuticals and Cosmetics Act of 1940 and the Rules of 1945.
Who is the Consultant for the MD 42 License?
ELT Consultancy is one of the best consultancies in the world, as they have all the experienced members on their team.
ls Medical Devices Online Without an MD 42 Registration?
No, you can not sell Medical Devices without an MD 42 license.
What Is The MD 42 License Used For?
The MD 42 License is used to legally sell, stock, exhibit, or distribute medical devices on a wholesale basis in India.
It is issued by the CDSCO or the State Licensing Authorities after approval of the MD 41 application.

